Updated Recommendations for Adult Pneumococcal Vaccination
Printable Updated Recommendations for Adult Pneumococcal Vaccination [PDF - 458 KB]
July 5, 2022
Summary of recommendations
On October 20, 2021, the Advisory Committee on Immunization Practices (ACIP) simplified adult pneumococcal vaccination recommendations across age and risk groups, now including people 19-64 years who have any of a broader group of chronic medical conditions and incorporating use of either 20-valent (PCV20) or 15-valent (PCV15) pneumococcal conjugate vaccines (PCV). Both PCV15 and PCV20 were licensed in 2021 for adults aged ≥18 years and expanded pneumococcal serogroup coverage for adults. This recommendation does not affect the pneumococcal vaccine schedule for children ≤18 years.
American Indians and Alaska Natives (AI/AN) are disproportionately affected by invasive pneumococcal disease and experience higher levels of disease and outbreaks in Tribal communities.5,6,7 Adopting the newest ACIP PCV recommendations may further prevent an additional 30% of invasive pneumococcal disease cases.9 ACIP outlines two distinct PCV immunization strategies and did not make a preferential recommendation for either among AI/AN individuals.
Recommendations for vaccine use
Vaccine Information Statement (VIS) [PDF]
Dosing, Timing & Administration (Algorithm for PCV15/PPSV23 and PCV20 Pneumococcal Immunization in Appendix B)
Adults aged 19–64 years with certain chronic diseases and immunocompromising conditions and adults aged ≥65 years who have not previously received any PCV or whose vaccination history is unknown should receive 1 dose of either PCV20 or PCV15.
- When PCV15 is used, it should be followed with one dose of PPSV23 at least one year later. A minimum interval of
8 weeks can be considered for adults with an immunocompromising condition.
- When PCV20 is used, no additional pneumococcal vaccine doses are recommended.
Adults who have previously received PPSV23 and have not received a pneumococcal conjugate vaccine may receive one dose of either PCV20 or PCV15 at least one year after their last PPSV23 dose. When PCV15 is used in those with a history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
Adults who previously received PCV13 should complete the previously recommended series with PPSV23 (with an interval of one year between doses for adults ≥65 years, or 8 weeks between doses for immunocompromised individuals), or one dose of PCV20 may be used if PPSV23 is not available.
Efficacy1
The FDA authorized PCV20 based on immunobridging studies involving the shared serotypes between PCV13 and PPSV23. PCV20 was non-inferior to all serotypes in common with PCV13 and 6 of the 7 serotypes that overlap with PPSV23. PCV15 was compared to PCV13 in clinical trials and found to be non-inferior for the 13 shared serotypes. Note that PCV20 protects against serotype 12F, which has been identified as a contributor to invasive pneumococcal disease among AI/AN, typically impacting children with subsequent transmission to elders. PPSV23 also provides coverage against serotype 12F; however, clinicians should assess the risk of waiting one year between doses for individuals if the PCV15 and PPSV23 series strategy is implemented (note: for immunocompromised patients this timeframe can be reduced to 8 weeks).
Administration
In alignment with CDC and ACIP recommendations, simultaneously administering all vaccines for which a person is eligible at the time of a visit increases the probability that an individual will be up to date on vaccinations. Clinicians should adhere to vaccine schedules and observe the appropriate minimum intervals between products when used in series.
Contraindications
Pneumococcal vaccines should not be administered to persons with a history of a severe allergic reaction, such as anaphylaxis, to any component of the vaccines, or to individuals with a previous allergic reaction to a dose of the same formulation of pneumococcal vaccine or diphtheria toxoid (in the case of PCV15).
Precautions
- Vaccination should be delayed for patients experiencing moderate or severe acute illness.
- Immunocompromised individuals may have a diminished immune response to pneumococcal vaccinations.
Storage and handling
All pneumococcal vaccines (PCV15, PCV20, and PPSV23) should be stored in a refrigerator at 2°C to 8°C. PCV15 and PCV20 require resuspension via vigorous shaking to ensure a homogenous white suspension prior to administration. Vaccinators should be particularly attentive to inspecting for large particulate matter and discoloration. Multiple attempts may need to be made to re-suspend the vaccine. PCV20 syringes should be stored horizontally to minimize resuspension time.
Availability, handling, and supply
All ACIP recommended vaccines are on the National Core Formulary. PCV20 is manufactured by Pfizer, PCV15, and PPSV23 are manufactured by Merck. All three pneumococcal vaccines are available under the Veterans Administration pricing contract under the IHS Prime Vendor, McKesson.
Adverse reactions and reporting
Post-vaccination adverse reactions that are mild to moderate in nature are common with pneumococcal vaccinations, particularly pneumococcal conjugate vaccines. Injection site reactions such as pain, redness, or swelling at the injection site are common. Systemic adverse reactions, such as myalgia, fatigue, headache, and joint pain may occur. Adverse events following administration of PCV20 or PCV15 were similar to those of PCV13 in all age groups, except for increased pain or swelling at the injection site for PCV15.11,12
Adverse events following vaccination should be reported according to local policy and to the Vaccine Adverse Events Reporting System (VAERS). For VAERS reporting within the IHS [PDF], including Tribal and Urban facilities, healthcare providers are requested to add “IHS” in item #26 for ongoing vaccine safety evaluation among the IHS patient population.4
RPMS-EHR documentation and forecasting
The pneumococcal schedule and guidance is complex and the forecaster may not identify all patients with their appropriate pneumococcal needs or timing.
- For adults aged ≥65 years: IHS EHR forecaster logic is currently age-based and already exists.
- Additional forecaster logic may be found here: Pneumococcal Vaccine Group - ICE
- Patients 19-64 years old should be identified through clinical review and consideration.
- For immunocompromised individuals, the forecaster does not currently assess for immunocompromising conditions; however, it will forecast PPSV23 for individuals with chronic underlying conditions as part of the “high-risk” forecaster, which includes individuals with diabetes, chronic heart disease, chronic lung disease, asthma, and smokers.
- The high-risk forecaster may be useful for identifying individuals with indications for PCV vaccine. However, if a PCV vaccine is given, the PPSV23 forecaster will not turn off.
- Future enhancements of the forecaster are in planning stages, which may help identify a patient’s pneumococcal vaccine needs.
- The accompanying flow chart may be used as a clinic reference guide (See Appendix B).
Additional information
| CPT | CVX | CPT Name | CPT Description |
|---|---|---|---|
| 90732 | 33 | Pneumococcal polysaccharide PPV23 | Pneumococcal polysaccharide vaccine, 23-valent (PPSV23), adult or immunosuppressed patient dosage, when administered to individuals 2 years or older, for subcutaneous or intramuscular use |
| 90671 | 215 | Pneumococcal conjugate PCV15, polysaccharide CRM197 conjugate, adjuvant, PF | Pneumococcal conjugate vaccine, 15 valent (PCV15), for intramuscular use |
| 90677 | 216 | Pneumococcal conjugate PCV20, polysaccharide CRM 197 conjugate, adjuvant, PF | Pneumococcal conjugate vaccine, 20 valent (PCV20), for intramuscular use |
Appendix A: Considerations for product selection
Cost-Effectiveness1
Compared to previous pneumococcal recommendations and, as discussed in the MMWR , economic models found cost savings in all scenarios and age groups for the use of either PCV20 alone or PCV15 in series with PPSV23. Clinics may opt for either strategy. See table below for acquisition cost per dose and series based on CDC public sector vaccine pricing .
| Vaccine | Cost per dose | Cost per series |
|---|---|---|
| PCV20 | $173.69 (single dose regimen) | $173.69 |
| pcV15 + PPSV23 (in series) | $149.90 (PCV15) + $74.50 (PPSV23) | $224.40 |
Other Considerations
Of note, PCV15 clinical trials intentionally enrolled a higher proportion of AI/AN individuals than are typically represented in the U.S. population. The number of AI/AN individuals was too small to draw any conclusions regarding safety and efficacy in AI/AN people and therefore should not be weighted in product selection.
Simplicity may be an important factor to consider in selecting vaccine products and local vaccination strategies. The number of doses in a series, minimum intervals between doses, risk for administration errors, and complexity of decision-making based on age or risk factors may be important considerations. As the number of vaccine doses in a series increases, series completion rates and adherence to dosing schedules become more challenging .13 It is more logistically challenging to administer different vaccines in series, there is a delay in serotype coverage due to series intervals (i.e. spacing between PCV15 and PPSV23), or lower serotype coverage overall if the series is not completed13. Multi-dose vaccine series require more clinical decision making, additional clinic scheduling, supplies for additional doses, and increased indirect costs to patients (e.g., time away from work, travel to the clinic, and management of post-vaccination side effects after each dose). From an inventory and procurement perspective, stocking, ordering, and managing multiple products increases administrative complexities and workload.
Additional consideration may be given to vaccine type: polysaccharide versus conjugate. PPSV23 is a polysaccharide vaccine that does not elicit immune memory cells, and efficacy may wane in 5-10 years.8 Conjugated vaccines induce memory B cells to enhance the immune response.10 In the case of PCV20, the conjugated vaccine may provide improved protection against the 5 serotypes that overlap between PPSV23 and PCV20, which are not otherwise covered by PCV15. Alternatively, PPSV23 provides coverage against 4 serotypes not covered by PCV20. These 4 serotypes account for only 8% of invasive pneumococcal cases in adults ≥ 65 years and 13-15% in individuals aged 19-64 years.9
For the Pneumococcal Algorithm see Appendix B after the References.
References
- Use of 15-Valent Pneumococcal Conjugate Vaccine and 20-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, 2022 | MMWR (cdc.gov)
- Pneumococcal Vaccine Timing for Adults
- Recommended Adult Immunization Schedule (cdc.gov) [PDF]
- Reporting a Suspected Vaccine Adverse Event. Indian Health Service, National Pharmacy and Therapeutics Committee. Accessed online 3.3.22 [PDF]
- Watt JP, O'Brien KL, Benin AL, et al. Risk factors for invasive pneumococcal disease among Navajo adults. Am J Epidemiol. 2007;166(9):1080-1087. doi:10.1093/aje/kwm178
- Bliss SJ, Larzalere-Hinton F, Lacapa R, et al. Invasive pneumococcal disease among White Mountain Apache adults, 1991-2005. Arch Intern Med. 2008;168(7):749-755. doi:10.1001/archinte.168.7.749
- Kobayashi M. Evidence to Recommendation Framework: Use of 15-valent and 20-valent Pneumococcal Conjugate Vaccines in Adults. Advisory Committee on Immunization Practices. Live meeting 6.25.2021. ACIP Kobayashi June 2021 Presentation Slides | Immunization Practices | CDC. [PDF]
- Hall E, Wodi AP, Hamborsky J, et al. Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th ed. Washington, D.C. Public Health Foundation, 2021. Chapter 17: Pneumococcal Disease. Accessed online 4.15.22
- Gierke R. Current Epidemiology of Pneumococcal Disease, United States – 2019 updates. Advisory Committee on Immunization Practices. Live meeting 6.25.2021. ACIP Gierke Oct 2021 Presentation Slides | Immunization Practices | CDC. [PDF]
- Kobayashi M. Considerations for Age-Based and Risk-Based Use of PCV15 and PCV20 among U.S. Adults and Proposed
Policy Options. Advisory Committee on Immunization Practices. Live meeting 10.20.2021. ACIP Kobayashi Oct 2021 Presentation Slides | Immunization Practices | CDC. [PDF]
- Prescribing information Prevnar 20 (Pneumococcal 20-valent Conjugate Vaccine). Revised 6/2021. Accessed online 4.15.22
- Prescribing information Vaxneuvance (Pneumococcal 15-valent Conjugate Vaccine). Accessed online 4.15.22
- Nelson JC, Bittner RC, Bounds L, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a vaccine safety datalink study. Am J Public Health. 2009 Oct;99 Suppl 2(Suppl 2):S389-97. doi: 10.2105/AJPH.2008.151332. PMID: 19797753; PMCID: PMC4504385.
If there are any questions regarding this document, please email the National Immunization Program at ImmunizationAdmins@ihs.gov.
Appendix B
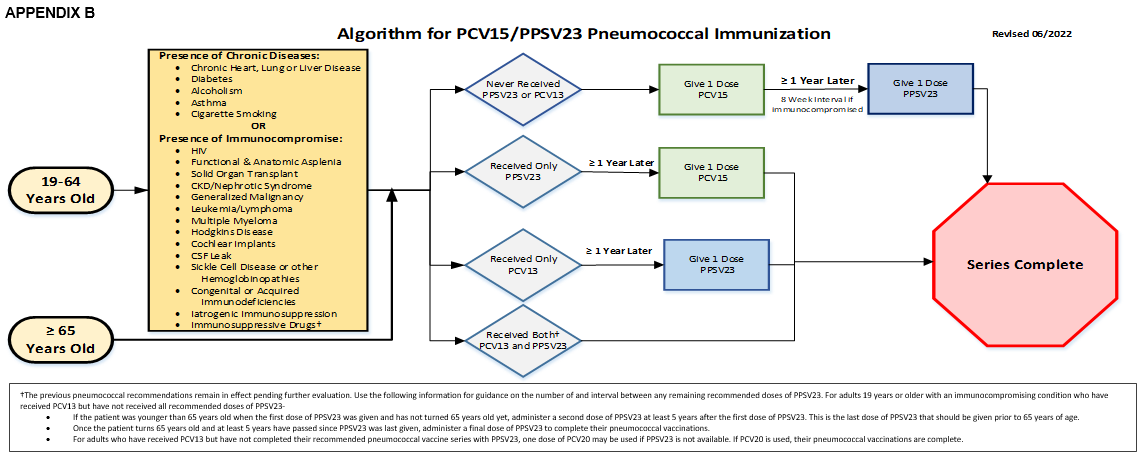
For all ages 19 and above:
- If they have never received PPSV23 or PCV13, they should:
- Receive one dose of PCV15, then
- Receive one dose of PPSV23 within a year or in 8 week interval if immunocompromised
- If they receive only PPSV23 then within a year
- Receive one dose of PCV15
- If they receive only PCV13, then within a year
- Receive one dose of PPSV23
- If they receive both PCV13 and PPSV23 then the
- Series is complete
†The previous pneumococcal recommendations remain in effect pending further evaluation. Use the following information for guidance on the number of and interval between any remaining recommended doses of PPSV23. For adults 19 years or older with an immunocompromising condition who have received PCV13 but have not received all recommended doses of PPSV23-
- If the patient was younger than 65 years old when the first dose of PPSV23 was given and has not turned 65 years old yet, administer a second dose of PPSV23 at least 5 years after the first dose of PPSV23. This is the last dose of PPSV23 that should be given prior to 65 years of age.
- Once the patient turns 65 years old and at least 5 years have passed since PPSV23 was last given, administer a final dose of PPSV23 to complete their pneumococcal vaccinations.
- For adults who have received PCV13 but have not completed their recommended pneumococcal vaccine series with PPSV23, one dose of PCV20 may be used if PPSV23 is not available. If PCV20 is used, their pneumococcal vaccinations are complete.
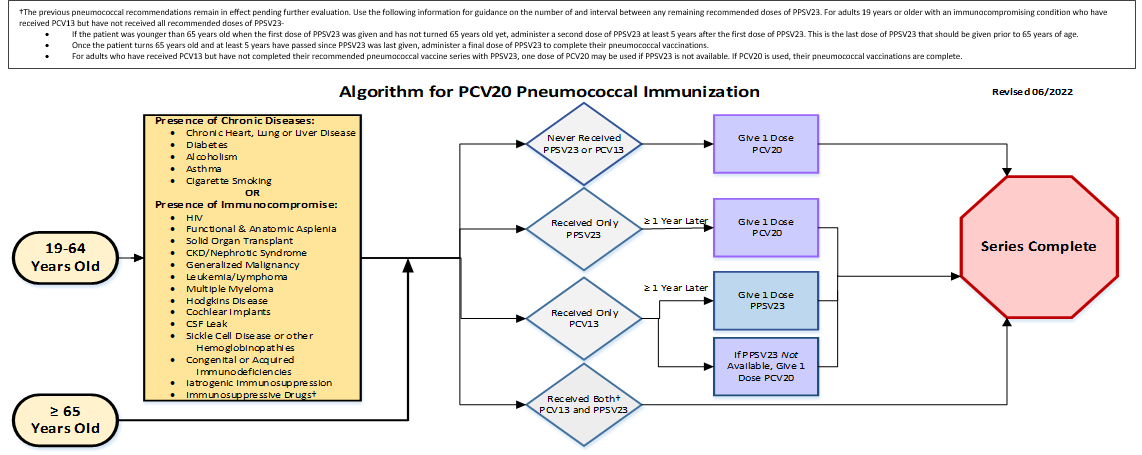
For all ages 19 and above:
- If patient has never received PPSV23 or PCV13, they should:
- Receive one dose of PCV20
- If patient has received only PPSV23, they should:
- Receive one dose of PCV20 within 1 year later
- If patient has received only PCV13, they should:
- Receive one dose of PPSV23 within 1 year later
- If PPSV23 not available, give 1 dose of PCV20
- Received both PCV13 and PPSV23 then the
- Series Complete


