Pharmacy Based COVID Therapeutics

Collaborative Practice Agreement
Gallup Indian Medical Center
March 2022
Chief of Medical Director Date
COVID Therapeutics Medical Director Date
Chief Pharmacist Date
Physicians entering into this Collaborative Practice Agreement
|
Institution Name: Gallup Indian Medical Center |
Date: ☐ New CPA ☐ Renewal |
|
Title of CPA (Areas of Care): COVID Therapeutics |
|
|
Collaborating Physician Physician Name: Alithea Gabrellas |
- Statement of Need
During COVID-19 peaks, providers at GIMC formed a Therapeutics Team to streamline treatments for patients testing positive. As the peak wanes, providers are needed back in primary clinics to resume seeing patients. Pharmacy has been asked to pick up the review of these patients to ensure expedited treatment for those testing positive for COVID-19.
- Purpose and Goals
To reduce morbidity and mortality from COVID-19 by providing timely antiviral treatment, as authorized by the Food Drug Administration.
- Clinic Procedures:
- Eligibility: Positive COVID test
- Patients identified as having positive laboratory test will automatically populate in iCare Therapeutics group.
- Patients identified as positive by home test will be reported to Contract tracers. The Contact tracers will contact Therapeutics team for patients reporting positive test who are symptomatic, ≥ 18 years of age and have symptom onset within past 7 days (first day of symptoms= day 0)
- iCare monitoring and chart review
- Designated pharmacist will repopulate iCare Therapeutics list on a regular basis (approximately every 1-2 hours) during their shift (usually between 8am-5pm).
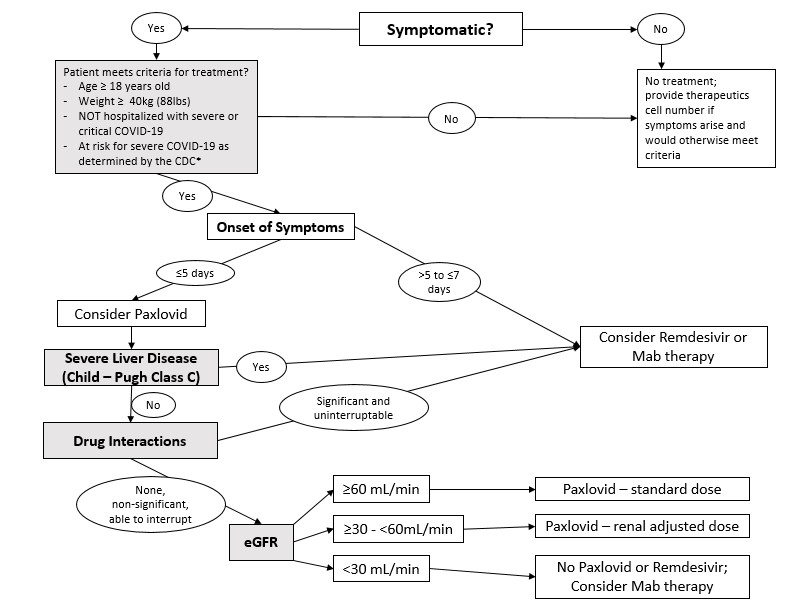
- Designated pharmacist will perform chart evaluation and screen for contraindications to COVID therapeutics to determine appropriate therapy. Pharmacist will screen for the following items to determine eligibility for therapy. See algorithm for treatment selection (below).
- Presence of symptoms
- Age ≥ 18 years
- Weight ≥ 40 kg
- Mild or Moderate COVID (no new hypoxia)
- Risk for severe COVID-19 as determined by the CDC list People with Certain Medical Conditions
- Onset of symptoms
- Presence of Liver Disease (Child-Pugh Score)
- Drug Interactions using Liverpool COVID-19 Drug Interaction Checker or Lexicomp
- Kidney Disease (eGFR)
- Appropriate therapy will be selected from the following based on treatment algorithm. Therapies are listed in order of preference.
- Paxlovid (nirmatrelvir/ritonavir)
- 5 day oral therapy
- Symptom onset within 5 days (first day of symptoms = day 0)
- Mild/Moderate COVID (no new hypoxia)
- No significant drug interaction with Paxlovid
- Child – Pugh Class C contraindicated with Paxlovid
- eGFR ≥60 : no adjustments necessary
- eGFR ≥30 to <60 :
- renal adjustment required for Paxlovid
- eGFR <30 or dialysis:
- no Paxlovid
- Veklury (remdesivir)
- 3 day infusion therapy
- FDA approved
- Symptom onset within 7 days
- Not hospitalized for COVID
- Risk of transaminase elevations with Veklury
- Do not begin if ALT >10x ULN within last 7 days
- eGFR ≥30 : no adjustments necessary
- eGFR <30:
- no Veklury
- Bebtelovimab
- 1 day IV infusion therapy
- Mild/Moderate COVID (no new hypoxia)
- Symptom onset within 7 days
- Only authorized for use in patients for whom no other FDA authorized therapy is appropriate or accessible
- Paxlovid (nirmatrelvir/ritonavir)
- Patient Visits
- Visits will be conducted by telephone contact.
- A COVID therapeutics pharmacist will be available Monday – Friday 8:30am-5pm
- If pharmacist deems Paxlovid is appropriate for given patient:
- Pharmacist will call patient to offer Paxlovid.
- If patient is not yet aware of their COVID test result:
- Make patient aware of result
- Briefly discuss isolation requirements
- Briefly discuss testing of close contacts. Contacts should test immediately and again at day 5-7 after exposure or sooner if symptoms develop.
- If patient is not yet aware of their COVID test result:
- Pharmacist will confirm and document date of onset of symptoms. Must be within 5 days to continue with Paxlovid (first day of symptoms = day 0).
- Pharmacist will confirm current medication list including herbal and OTC products to ensure no drug interactions have been missed.
- Pharmacist must counsel patient about Paxlovid using the information from the EUA and Paxlovid Fact Sheet
- Counsel patient using Paxlovid Fact Sheet
- Counsel on drug interactions
- Counsel on common side effects (nausea)
- If patients agrees to treatment:
- Pharmacist will contact COVID Therapeutics provider to place Paxlovid order:
- Standard dosing: 300mg (2 tablets) nirmatrelvir and 100mg (1 tablet) ritonavir by mouth at the same time twice daily for 5 days. (qty 30 tablets)
- Renal impairment dosing (eGFR >30 to <60ml/min): 150mg (1 tablet) nirmatrelvir and 100mg (1 tablet) ritonavir by mouth at the same time twice daily for 5 days. (qty 20 tablets)
- Provider will also enter orders for ondansetron for nausea:
- Ondansetron 4mg: 1 tablet by mouth every 8 hours as needed to prevent nausea; #10
- Provider will call Main pharmacy at ext: 71797 to ensure order is expedited.
- Patient will be instructed by Pharmacist on how to pick up medication at the Main pharmacy.
- Pharmacist will contact COVID Therapeutics provider to place Paxlovid order:
- If patient refuses treatment or is currently asymptomatic:
- Pharmacist will provide patient with COVID therapeutics cell number in the event that they later develop symptoms and/or decide they would like treatment.
- Pharmacist will document patient interaction in patients EHR using COVID-19 therapeutics note. Alithea Gabrellas and patients PCP (if patient has one) will be co-signed on the note.
- Pharmacist will call patient to offer Paxlovid.
- If pharmacist deems Veklury (remdesivir) is appropriate for given patient:
- Pharmacist will call patient to offer Veklury.
- If patient is not yet aware of their COVID test result:
- Make patient aware of result
- Briefly discuss isolation requirements
- Briefly discuss testing of close contacts. Contacts should test immediately and again at day 5-7 after exposure or sooner if symptoms develop.
- If patient is not yet aware of their COVID test result:
- Pharmacist will confirm and document date of onset of symptoms. Must be within 7 days (first day of symptoms = day 0).
- Pharmacist will confirm current medication list including herbal and OTC products to ensure no drug interactions have been missed.
- Pharmacist will counsel patient about Veklury based on information found in the prescribing information.
- Counsel patients concerning need for 3 consecutive visits to the hospital for infusion.
- Ensure patient will have transport for all 3 days
- Counsel on common side effects (nausea, infusion site reactions)
- Counsel patients concerning need for 3 consecutive visits to the hospital for infusion.
- If patients agrees to treatment:
- Pharmacist will place referral to infusion clinic
- Infusion clinic will call patient to set up appointment but if they miss the call they infusion clinic can be reached at 505-722-1553.
- Pharmacist will place order for required baseline labs to be completed at first infusion appointment.
- CMP
- PT & PTT
- Pharmacist will call provider to place order in EHR for Veklury (remdesivir).
- Pharmacist will place referral to infusion clinic
- If patient refuses treatment or is currently asymptomatic:
- Pharmacist will provide patient with COVID therapeutics cell number in the event that they later develop symptoms and/or decide they would like treatment.
- Pharmacist will document patient interaction in patients EHR using COVID-19 therapeutics note. Alithea Gabrellas and patients PCP (if patient has one) will be co-signed on the note.
- Pharmacist will call patient to offer Veklury.
- If pharmacist deems bebtelovimab is appropriate for given patient:
- Pharmacist will call patient to offer bebtelovimab infusion.
- If patient is not yet aware of their COVID test result:
- Make patient aware of result
- Briefly discuss isolation requirements
- Briefly discuss testing of close contacts. Contacts should test immediately and again at day 5-7 after exposure or sooner if symptoms develop.
- Pharmacist will confirm and document date of onset of symptoms. Must be within 7 days (first day of symptoms = day 0).
- Pharmacist will confirm current medication list including herbal and OTC products to ensure no drug interactions have been missed.
- Pharmacist must counsel patient about bebtelovimab based on information found in EUA Fact Sheet.
- Counsel patients concerning need to go to the hospital for infusion.
- Ensure patient will have transport
- Counsel on common side effects (nausea, infusion related reactions)
- Counsel patients concerning need to go to the hospital for infusion.
- If patients agrees to treatment:
- Pharmacist will place referral to infusion clinic
- Infusion clinic will call patient to set up appointment but if they miss the call they infusion clinic can be reached at 505-722-1553.
- Pharmacist will call provider to place order in EHR for bebtelovimab and PRN meds.
- Pharmacist will place referral to infusion clinic
- If patient refuses treatment or is currently asymptomatic:
- Pharmacist will provide patient with COVID therapeutics cell number in the event that they later develop symptoms and/or decide they would like treatment.
- Pharmacist will document patient interaction in patients EHR using COVID-19 therapeutics note. Alithea Gabrellas and patients PCP (if patient has one) will be co-signed on the note.
- If patient is not yet aware of their COVID test result:
- Pharmacist will call patient to offer bebtelovimab infusion.
- If the workload goes above the capacity of the Pharmacy team due to a COVID-19 surge and availability of treatment, Pharmacy will inform Incident Command and/or Executive Leadership Team about the need for additional support to successfully treat all eligible patients in a reasonable timeframe. Additional staff from other departments will assist as deemed appropriate for the workload based on a tiered system developed by Pharmacy.
- Eligibility: Positive COVID test
- Laboratory Authority:
The Covid therapeutics Clinic Pharmacists may order and interpret laboratory data for treating the following diseases/conditions:
- COVID-19 infection
- Renal and hepatic dosing will be based on most recent lab values available. If any question, the pharmacist will consult the overseeing provider.
Prescriptive Authority:
- Eligibility/Treatment decision tree
- Training/Certification
Physician with collaborative responsibilities will conduct clinical competency assessments.
Pharmacist must:
- Attest to review of current EUA for each medication
- Attest to review of GIMC current policies for each medication
- ASHP CE . Certificate of completion must be provided.
- Completion of Paxlovid Competency and passing with at least 85%.
- Outcome Measures
Peer-review/-evaluation based on latest guidelines will be assessed by an infectious disease physician regularly. The results will be reviewed with the pharmacists and recommendations made for improvement if needed.


 View Larger Image
View Larger Image