Update on Dental Amalgam
Minamata Convention on Mercury Annex A, Part II: Products subject to Article 4, paragraph 3
Mercury-added products: Dental amalgam
Provisions
Measures to be taken by a Party to phase down the use of dental amalgam shall take into account the Party’s domestic circumstances and relevant international guidance and shall include two or more of the measures from the following list:
(i) Setting national objectives aiming at dental caries prevention and health promotion, thereby minimizing the need for dental restoration;
(ii) Setting national objectives aiming at minimizing its use;
(iii) Promoting the use of cost-effective and clinically effective mercury-free alternatives for dental restoration;
(iv) Promoting research and development of quality mercury-free materials for dental restoration;
(v) Encouraging representative professional organizations and dental schools to educate and train dental professionals and students on the use of mercury-free dental restoration alternatives and on promoting best management practices;
(vi) Discouraging insurance policies and programmes that favour dental amalgam use over mercury-free dental restoration;
(vii) Encouraging insurance policies and programmes that favour the use of quality alternatives to dental amalgam for dental restoration;
(viii) Restricting the use of dental amalgam to its encapsulated form;
(ix) Promoting the use of best environmental practices in dental facilities to reduce releases of mercury and mercury compounds to water and land.
IHS Dental Amalgam Usage Data
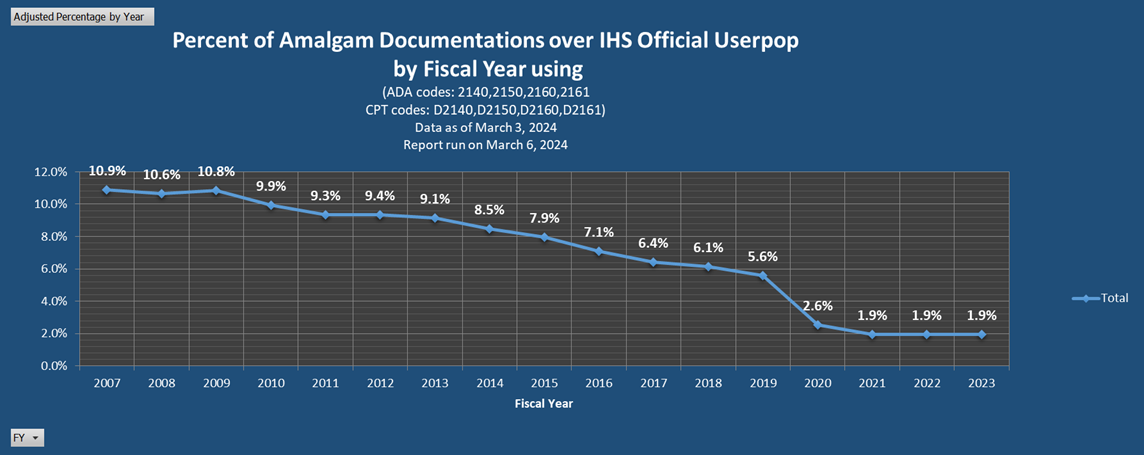
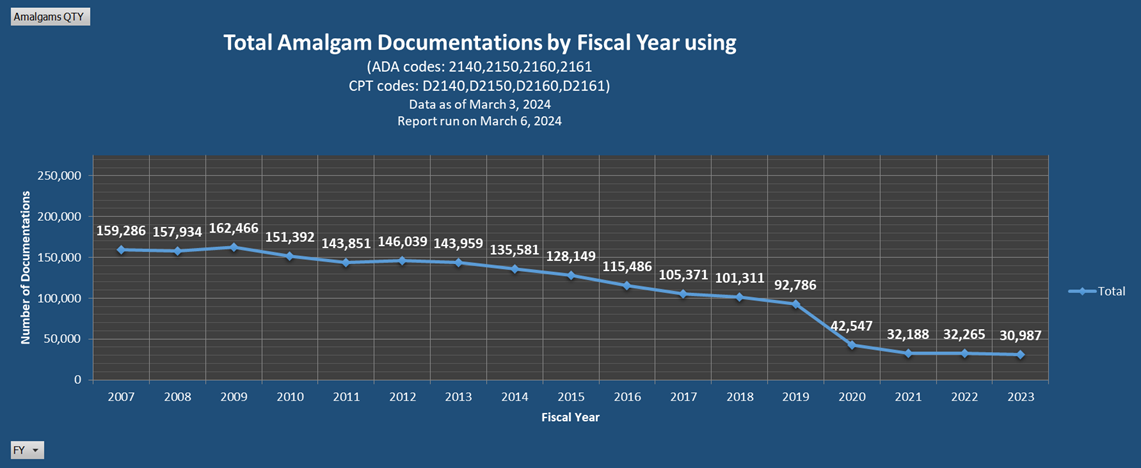
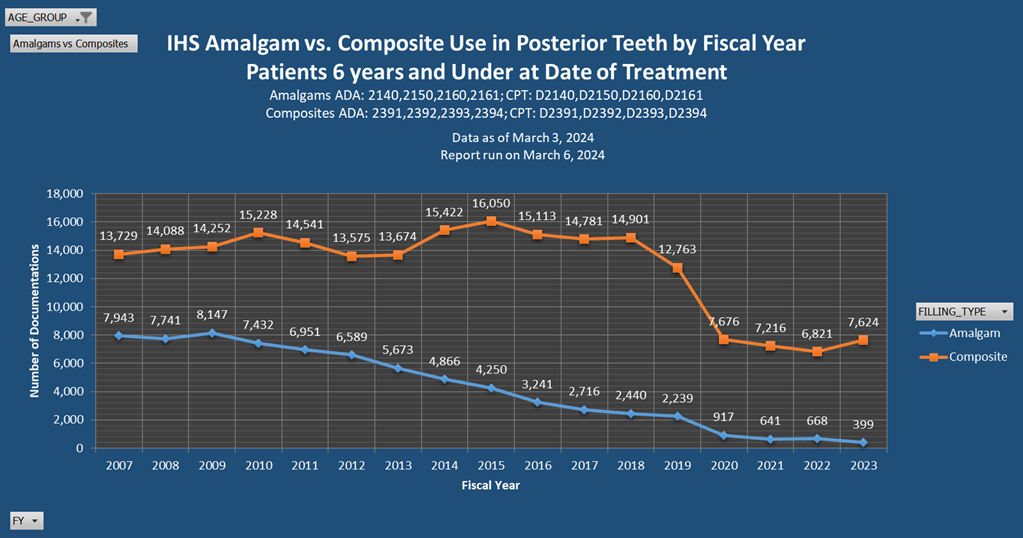
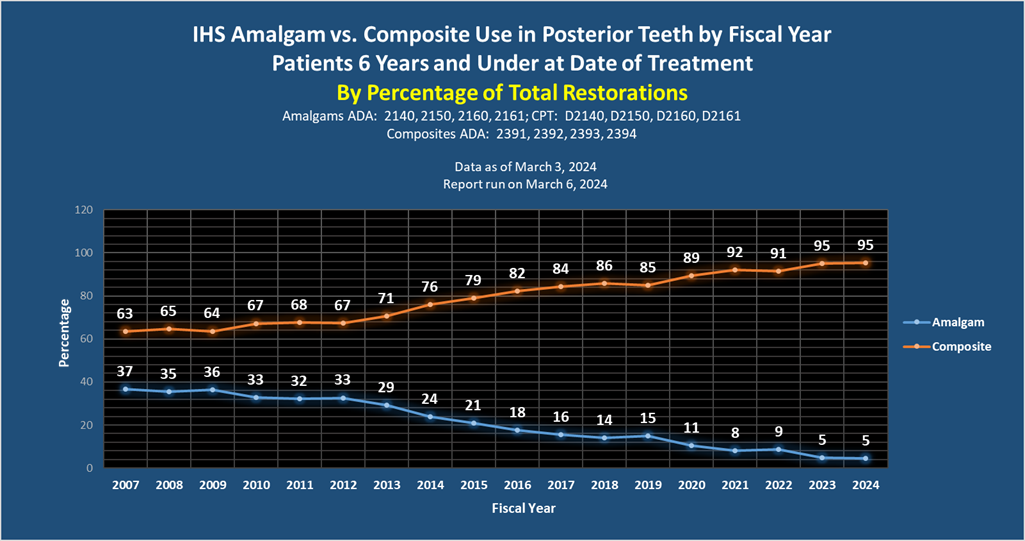
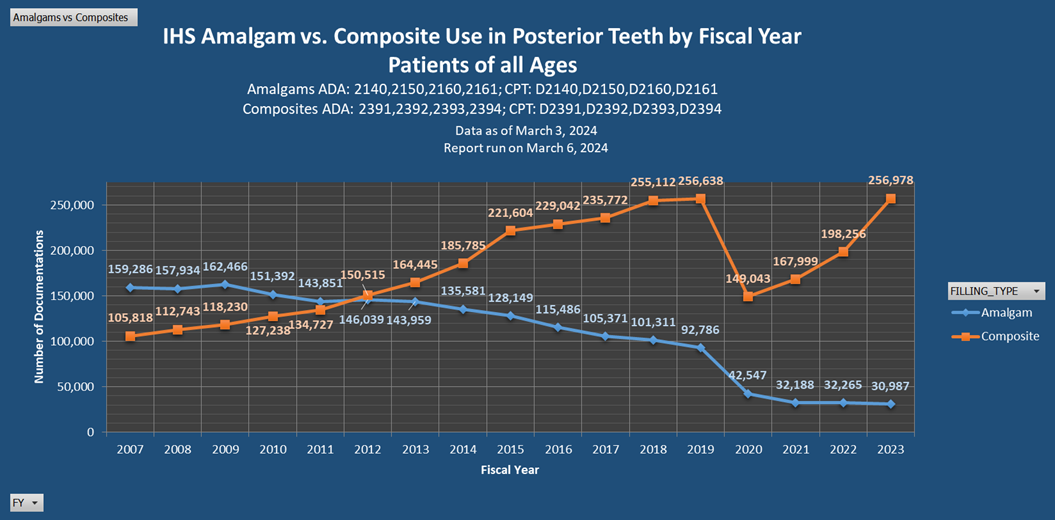
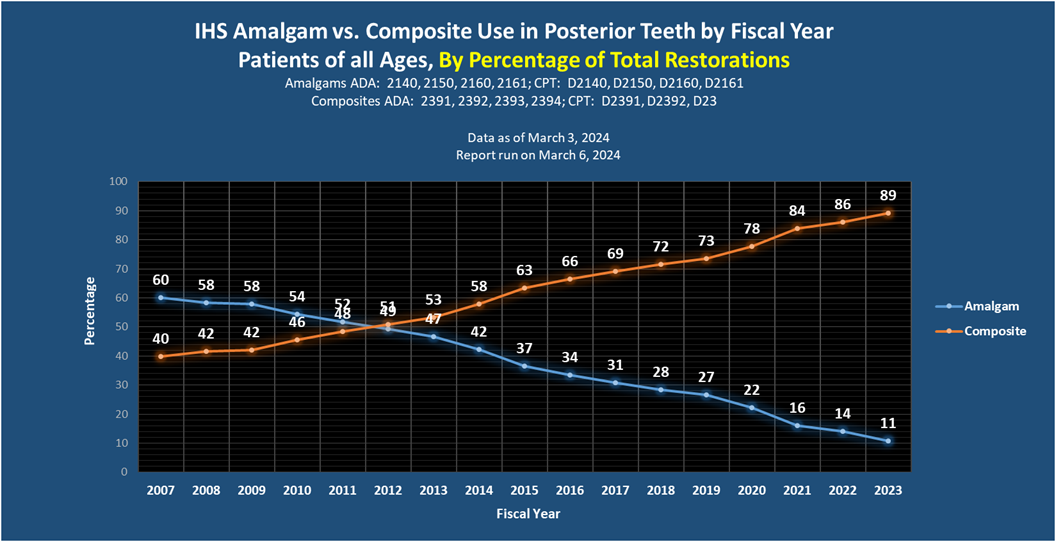
Additional Resources
IHS Division of Oral Health Dental Amalgam Phase-Down Plan, 2010-2040 IHS DOH Dental Amalgam Phase-Down Plan 2010-2040 (Updated 6-2024) (PDF - 596KB)
US FDA – Dental Amalgam Fillings https://www.fda.gov/medical-devices/dental-devices/dental-amalgam-fillings
US FDA – Information for Patients About Dental Amalgam Fillings https://www.fda.gov/media/142415/download
US EPA - Frequently Asked Questions on the Dental Office Category Rule https://www.epa.gov/sites/production/files/2017-12/documents/dental-office-category_frequent-questions_nov-2017.pdf
Minamata Convention on Mercury – Text and Annexes https://www.mercuryconvention.org/sites/default/files/2021-06/Minamata-Convention-booklet-Sep2019-EN.pdf
Conference of the Parties to the Minamata Convention on Mercury, Fourth meeting, Information on Dental Amalgam https://www.mercuryconvention.org/sites/default/files/documents/working_document/4_5_DentalAmalgam.English.pdf



