Albuquerque Area T2T Best Practices
I. Identification
- AAO COVID-19 Test-to-Treat Approach With Paxlovid™
II. Policy or Purpose Statement
- During COVID-19 surge, Medical and/or Pharmacy Clinic Provider within the Albuquerque I/T/U Healthcare facilities may need to streamline treatments for patients testing positive for Medical and/or Pharmacy Clinic Provider will review and arrange expedited treatment for those testing positive for COVID-19.
- To reduce morbidity and mortality from COVID-19 by providing timely antiviral treatment, as authorized by the U.S. Food and Drug Administration.
- To provide the Medical and Pharmacy Clinics with guidelines for treatment of mild to moderate COVID-19 with currently available oral antiviral therapies under Emergency Use Authorizations (EAU).
- COVID-19 Test to Treat: DHHS launched a nationwide Test to Treat initiative to give individuals an important new way to quickly access free lifesaving treatment for COVID-19. Through this program, people are able to get tested and – if they are positive and treatments are appropriate for them – receive a prescription from a health care provider, and have their prescription filled all at one location. These "One-Stop Test to Treat" sites are available at I/T/U healthcare facilities, including pharmacy-based clinics. People can continue to be tested and treated by their own health care providers who can appropriately prescribe these oral antivirals at locations where the medications are distributed.
III. Responsibilities
- The Chief Executive Officer or designee has the responsibility for overall compliance and enforcement with this policy.
- The Division/Department Directors or designee is responsible for this memorandum review of policy with end-users.
- The Clinical/Administrative Managers/Supervisor is responsible for educating end-users on the content of this policy and taking corrective action.
- Federal Healthcare facilities:
- Acoma-Canoncito-Laguna Service Unit
- Acoma-Canoncito-Laguna Service Indian Health Center
- Albuquerque Service Unit
- Albuquerque Indian Health Center
- Santa Ana Health Center
- Zia Health Center
- Jicarilla Service Unit
- Dulce Health Center
- Mescalero Service Unit
- Mescalero Indian Hospital
- Santa Fe Service Unit
- Santa Fe Indian Health Center
- Cochiti Health Center
- Santa Clara Health Center
- Taos Picuris Service Unit
- Taos Picuris Health Center
- Southern Colorado Service Unit
- Ute Mountain Ute Health Center
- Zuni Service Unit
- Zuni Indian Hospital
- Acoma-Canoncito-Laguna Service Unit
IV. Definitions
- Coronavirus: This common term used for the current virus actually describes a family of viruses that can affect humans and That family of viruses is responsible for the common cold, as well as more severe diseases such as SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East respiratory syndrome). More specifically, this virus has been named "SARS-CoV-2." The illness the virus causes is COVID-19, which stands for "Coronavirus Disease 2019." COVID-19 is the name of the disease, not the virus.
- Paxlovid™
- Paxlovid™ is an oral combination antiviral medication (nirmatrelvir/ritonavir) that can be taken at home to help keep high-risk patients from getting so sick that they need to be hospitalized.
- Paxlovid™ is an antiviral therapy that consists of two separate medications packaged When you take your three tablet dose, two tablets will be nirmatrelvir, which inhibits a key enzyme that the COVID virus requires in order to make functional virus particles. After nirmatrelvir treatment, the COVID virus that is released from the cells is no longer able to enter uninfected cells in the body, which, in turn, stops the infection. The other is ritonavir, a drug that is essentially shuts down nirmatrelvir's metabolism in the liver, so that it doesn't move out of your body as quickly, which means it can work longer giving it a boost to help fight the infection.
- Paxlovid™ has not been approved, but has been authorized for emergency use by FDA under an EUA, for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death.
- Paxlovid™ is an investigational medicine used to treat mild-to-moderate COVID-19 in adults and children [12 years of age and older weighing at least 88 pounds (40 kg)] with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death.
- Paxlovid™ is investigational because it is still being studied. There is limited information about the safety and effectiveness of using PAXLOVID to treat people with mild-to-moderate COVID-19.
V. Policy or Procedures (The Plan; The Guideline)
- Eligibility: Positive SARS-CoV-2, the virus that causes COVID-19.
- Patients identified as positive by in-home test will be reported to appropriate Contact Tracers and/or healthcare personnel.
- Contact Tracers and/or healthcare personnel will contact COVID-19 Case Manager for patients reporting positive COVID-19 test who are:
- ≥ 18 years of age and have symptom(s) onset within the past 5 days (first day of positive test or symptoms, whichever is first),
- 12-17 years of age may be a candidate for treatment if they have a serious medical condition,
- Patients particularly those at high risk do not have to have symptoms to be candidates for treatment, and/or
- Patient may self-report results of an in-home test to pharmacy via telephone. (It is recommended patients do not report (go to) to the healthcare facility in person if COVID-19 positive).
- Screening Chart review
- The Medical and/or Pharmacy Clinic Provider will perform chart evaluation and screen for contraindications to COVID therapeutics to determine appropriate therapy. Medical and/or Pharmacy Clinic Provider will screen for the following items to determine clinical consideration eligibility for therapy.
- Presence of symptoms (patient does not need to have symptoms to treat, but if they do have symptoms, onset should be within the past 5 days).
- Age ≥ 18 years
- 12-17 year olds may be candidates if they have a serious underlying medical
- Weight ≥ 40 kg (88lbs)
- Mild or Moderate COVID-19 signs and symptoms (no new hypoxia) not requiring hospitalization
- Risk for severe COVID-19 as determined by the CDC list People with Certain Medical Conditions. If a patient test positive and is an older adult or an individual who is at high risk of getting very ill from COVID-19, it is recommended for patient to contact a healthcare provider as soon as possible after a positive test to determine if the patient is eligible for Oral Antiviral therapeutics, even if symptoms are mild.
- Contraindications:
- Severe liver disease
- Paxlovid™ is not recommended in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2) until more data are available.
- Precautions:
- Hepatotoxicity
- Onset of symptoms
- No significant drug interactions with Paxlovid™
- Vaccination status
- Presence of symptoms (patient does not need to have symptoms to treat, but if they do have symptoms, onset should be within the past 5 days).
- Appropriate therapy will be selected from the following based on treatment algorithm.
- Paxlovid™ (nirmatrelvir/ritonavir)
- Contraindications
- History of severe hypersensitivity reaction to nirmatrelvir or ritonavir or any other components
- Chronic kidney: eGFR <30mL/min/1.73 m2
- Liver disease: Severe hepatic impairment (Child-Pugh Class C)
- Co-administration with medication dependent on CYP3A for clearance where elevated concentrations are associated with serious and/or life-threatening reactions
- Alfuzosin, pethidine, propoxyphene, anolazine, amiodarone, dronedarone, flecainide, propafenone, quinidine, colchicine, lurasidone, pimozide, clozapine, dihydroergotamine, ergotamine, methylergonovine, lovastatin, simvastatin, sildenafil, triazolam, oral midazolam
- Drug Interactions using Liverpool COVID-19 Drug Interaction Checker
- Co-administration with potent CYP3A inducers may decrease the potential for loss of virologic response and possible resistance.
- Apalutamide, carbamazepine, phenobarbital, phenytoin, rifampin, John's Wort
- Drug Interactions using Liverpool COVID-19 Drug Interaction Checker
- Precautions:
- Hepatotoxicity: Hepatic transaminase elevations, clinical hepatitis, and Use caution in patients with pre-existing liver diseases, liver enzyme abnormalities, or hepatitis.
- HIV-1 Drug Resistance: may lead to a risk of HIV-1 developing resistance to HIV protease inhibitors in individuals with uncontrolled or undiagnosed HIV-1
- Ritonavir can decrease the efficacy of combined hormonal
- Adverse reactions
- Commonly seen (1-2%) side effects in clinical trials include dysgeusia, diarrhea, hypertension, and myalgia.
- Dosing
- For eGFR >60 mL/min/1.73 m2:
- Nirmatrelvir 300mg (two 150mg tablets) with ritonavir 100mg (1 tablet) PO BID for 5 days
- For eGFR >30 to <60 mL/min/1.73 m2:
- Nirmatrelvir 150mg (1 tablet) with ritonavir 100mg (1 tablet) PO BID for 5 days
- For eGFR >60 mL/min/1.73 m2:
- The provider is responsible reporting of all serious adverse events and medication errors potentially related to Paxlovid™ via MedWatch.
- Contraindications
- Paxlovid™ (nirmatrelvir/ritonavir)
- Patient Visits
- Visits will be conducted by telephone
- A Medical and/or Pharmacy Clinic Provider will be available during normal operating patient care hours.
- If Medical and/or Pharmacy Clinic Provider deems Paxlovid™ is appropriate for given patient:
- Medical and/or Pharmacy Clinic Provider will call patient to offer Paxlovid™.
- If patient is not yet aware of their COVID test result:
- Make patient aware of result
- Briefly discuss isolation requirements
- Briefly discuss testing of close Contacts should test immediately and again at day 5-7 after exposure or sooner if symptoms develop.
- If patient is not yet aware of their COVID test result:
- Medical and/or Pharmacy Clinic Provider will confirm and document date of onset of symptoms. Must be within 5 days to continue with Paxlovid™ (first day of symptoms = day 0).
- Medical and/or Pharmacy Clinic Provider will confirm current medication list including herbal and OTC products to review drug interactions.
- Medical and/or Pharmacy Clinic Provider will counsel patient about Paxlovid™ using the information from the EUA and Paxlovid™ Fact Sheet (listed in the references)
- Counsel patient using Paxlovid™ Fact Sheet
- Counsel on drug interactions
- Counsel on common side effects (nausea)
- If patient agrees to treatment:
- Medical and/or Pharmacy Clinic Provider will create and sign an order for Paxlovid™ in the patient's health record.
- Standard dosing: 300mg (2 tablets) nirmatrelvir and 100mg (1 tablet) ritonavir by mouth at the same time twice daily for 5 days. (quantity 30 tablets)
- Renal impairment dosing (eGFR >30mL/min/1.73 m2 to <60 mL/min/1.73 m2): 150mg (1 tablet) nirmatrelvir and 100mg (1 tablet) ritonavir by mouth at the same time twice daily for 5 days. (quantity 20 tablets)
- Medical and/or Pharmacy Clinic Provider will create and sign an order for Paxlovid™ in the patient's health record.
- If patient refuses treatment:
- Medical and/or Pharmacy Clinic Provider will provide patient with central telephone number in the event that patient later decide they would like to participate in test to treatment program.
- Medical and/or Pharmacy Clinic Provider will document the patient encounter using COVID-19 Therapeutics Test-to-Treat TIU Reminder Dialog Patient's PCP (or designee) will be identified as the additional signer on the note.
- Medical and/or Pharmacy Clinic Provider will call patient to offer Paxlovid™.
- The Pharmacy Clinic Provider will refer patients for clinical evaluation with a medical clinician authorized under state law to prescribe drugs, if any of the following apply:
- If pharmacist deems not enough information is available to assess renal and hepatic
- If patient has a history of decreased renal function (eGFR <60 mL/min/1.73 m2) and no labs within 1 year refer to clinician.
- Not enough information is available to assess for any potential drug
- Modification of other medications is needed due to a potential drug
- Pharmacist may contact the clinician to discuss drug
- Paxlovid™ is not an appropriate therapeutic option based on the current Fact Sheet for Healthcare Providers, current risk level stratification, or due to potential drug interactions for which recommended monitoring would not be feasible.
- If pharmacist deems not enough information is available to assess renal and hepatic
- If the workload goes above the capacity of the Medical and/or Pharmacy Clinic Provider team due to a COVID-19 surge and availability of treatment, Medical and/or Pharmacy Clinic Provider will inform Clinical Executive Leadership about the need for additional support to successfully treat all eligible patients in a reasonable timeframe. Additional, staff from other departments will assist as deemed appropriate for the workload based on a tiered system.
- The Medical and/or Pharmacy Clinic Provider will perform chart evaluation and screen for contraindications to COVID therapeutics to determine appropriate therapy. Medical and/or Pharmacy Clinic Provider will screen for the following items to determine clinical consideration eligibility for therapy.
- Laboratory Authority:
- The COVID therapeutics Medical and/or Pharmacy Clinic Provider may order and interpret laboratory data for treating the following diseases/conditions:
- COVID-19 infection
- Renal and hepatic dosing will be based on most recent lab values available. If any question, the Pharmacy Clinic Provider will consult the overseeing Medical Clinic
- The COVID therapeutics Medical and/or Pharmacy Clinic Provider may order and interpret laboratory data for treating the following diseases/conditions:
- Training/Certification
- Attest to review of current EUA for
- Attest to review current policies for
- For Pharmacist:
- ASHP CE .
- Certificate of completion must be provided.
- Outcome Measures
- Peer-review and evaluation are based on latest guidelines will be assessed by the collaborating clinicians regularly. The results will be reviewed with the Medical and/or Pharmacy Clinic Provider and recommendations made for improvement if needed.
- Prescriptive Authority:
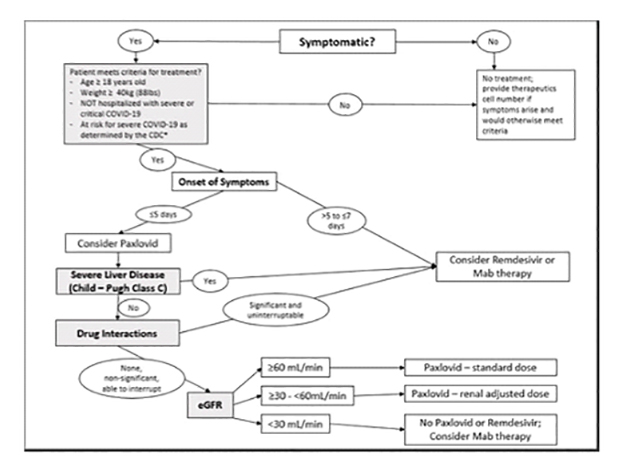
- Eligibility/Treatment decision tree (algorithm created by Gallup Indian Medical Center IHS)
VI. Source/Citation Reference(s)
- CDC: People with Certain Medical Conditions
- Paxlovid™ Fact Sheet for Providers
- Paxlovid™ Fact Sheet for Patients, Parents, and Caregivers
- FDA Authorizes Pharmacists To Prescribe PaxlovidPaxlovid™.pdf
VII. Scope
- Medical
- Pharmacy
VII. Reference(s) of Regulatory Agency Standard(s)
- TJC-Medication Management
- AAAHC-Pharmaceutical Services
IX. History/Supersedes
- Not Applicable
X. ReviewandApproval
- Approver(s):
- Area Chief Medical Officer
- Area Pharmacy Consultant
Applicability
Acoma-Canoncito-Laguna Service Unit, Albuquerque Area Office, Albuquerque IHS Dental Clinic, Albuquerque Service Unit, Jicarilla Service Unit, Mescalero Service Unit, New Sunrise Regional Treatment Center, Santa Fe Service Unit, Taos Picuris Health Center, Ute Mountain Ute Health Center, Zuni Service Unit


