Documenting Common ADEs
Common adverse events are the anticipated but unwanted experiences that patients have after taking a medication. These adverse events can be documented in the patient?s problem list as a purpose of visit. An Adverse Drug Event pick list has been created to easily find commonly reported adverse events. It is important that the name of the suspected medication be included in the purpose of visit narrative to associate the medication with the finding.
Documenting Common ADEs using the Integrated Problem List
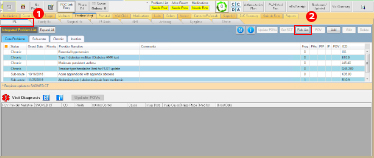
- In the RPMS-EHR, navigate to the Problem Management Tab of your RPMS-EHR and select the Integrated Problem List (IPL).
- Click the “Pick List” button.
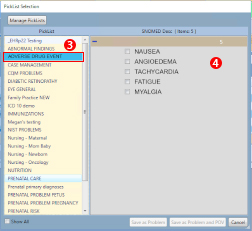
- Select the Adverse Drug Event Pick List and choose the adverse drug event you wish to document. If the adverse event does not appear in the pick list or if you would prefer to search for the appropriate term, you can search for the event the same way you search for other SNOMED terms.
A sample Adverse Drug Event pick is available in the RPMS-EHR. Talk with your Clinical Applications Coordinator (CAC) if you would like to add or modify options on the pick list. - Select the Adverse and add as a problem and POV as appropriate.
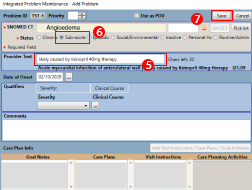
- Document the suspected cause of the Adverse Drug Event in the “Provider Text” field.
- Select sub-acute to identify it as a non-chronic condition.
- Click the “Save” button.