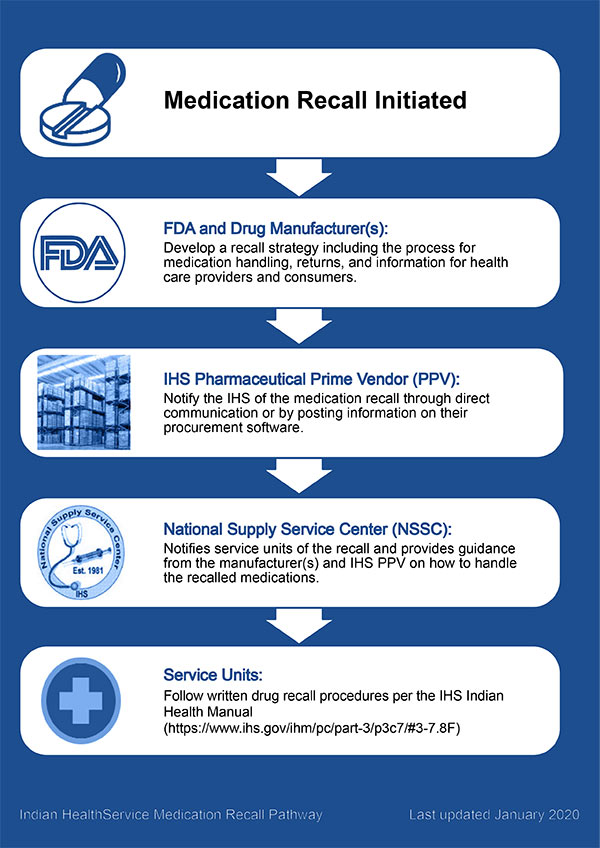
How to Handle Drug Recalls
Actions taken by a drug manufacturer and overseen by the FDA to remove a drug from the market to reduce the risk of adverse events. Recalls can occur for many reasons. Some recalls may result from minor labeling violations while others may occur due to impurities in the medication or a risk of life-threatening adverse events. Recent medication recalls can be found on the FDA website .
FDA Recall Letter Templates
Use these templates created in MS Word to inform your patients about any FDA Medication Recall that affects them.
FDA Recall: Do Not Stop Taking Medication FDA Recall: Stop Taking MedicationThe Medication Recall Process