Documenting Serious or Unexpected ADEs
- Open the Adverse Reaction Tracking system (ART), which is usually located on the home page of the RPMS-EHR.
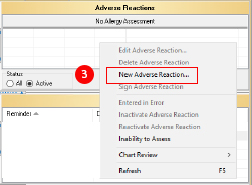
All serous and unexpected ADEs should be documented in the ART to prevent the event from occurring again by providing a warning if the medication is prescribed again. - Right click in the ART field.
- Select “New Adverse Reaction.” This will open the ART window.
- In the ART window:
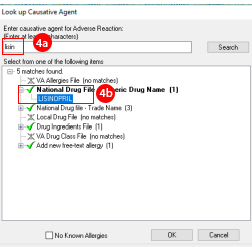
- Type the first few letters of the suspected medication that caused the adverse event
- Select it from the list.
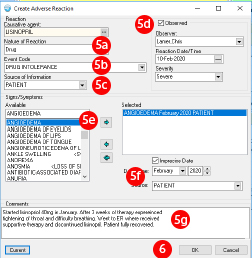
- Enter the information about the adverse drug event:
- Nature of reaction will default to drug since a medication was selected.
- Choose drug intolerance to identify the adverse drug event
- Source of Information is how you learned about the adverse drug event, this may often be from the patient
- Click the “Observed” checkbox if the adverse drug event was recent and observed. The clinician does not need to be the one who observed the event; a family member or the patient may have observed the event. If Observed is selected you will be prompted to enter the date of the adverse drug event and the severity.
If you do not check this box, the reaction is captured as a historical adverse drug event. - Select one or more signs or symptoms that occurred during the adverse drug event and use the arrow buttons to add or remove them from the selected box.
- You will be prompted to enter the date of the ADE again and identify the source.
- There is a free text comments field, which enables you to document additional information related to the ADE.
- Click “OK” to add the ADE to the patient?s record.
Frequently Asked Questions About Documenting in the ART
- Can you document non-serious ADEs in the ART?
- Yes. Any adverse drug event or allergy can be documented in the ART.
- What is a serious ADE?
- Serious Adverse Drug Events/Reactions are those that:
- Result in death
- Require hospital admission or prolongation of an existing hospital stay
- Result in persistent or significant disability/incapacity
- Are life threatening
- Cause congenital abnormalities or birth defects
- What is an unexpected ADE?
- Unexpected Adverse Drug Events/Reactions are those that occur but are not found labeled in the package insert, pharmacologic references, or in the medical literature or that occur in a patient where the effect is unexpected.


