Reporting ADEs to MedWatch
Submit an Adverse Drug Event report for a medication with the MedWatch reporting program. There are three options for submitting a MedWatch report:
- Complete the Online Voluntary Reporting Form on the FDA website.
- Complete the FDA 3500 form [PDF - 190 KB] and mail or fax the form to the FDA per the instructions on the form.
- For sites using the RPMS EHR, you can download the IHS Adverse Event EHR note template . This template will help craft your note and create a MedWatch submission at the same time. For sites not using RPMS EHR, a similar template can be developed.
When using the online voluntary Reporting Form or the FDA 3500 form, it is essential that the words "IHS" or "Indian Health Service" appear on the form, ideally in the address of the "Reporter" section.
Submit the ADE to the FDA MedWatch Program via the RPMS EHR Template
It is important that you document Indian Health Service on the form, either at the top or in the ”Reporter“ section.
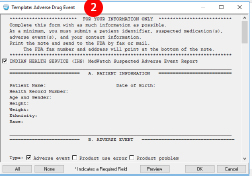
- To use the ADE template, create a new note with the Adverse Reaction/Allergy note title.
- Choose the Adverse Drug Event note template. The template will populate most of the required information to make filling out the form quick and easy. You will need to enter information related to the ADE such as the clinical course.
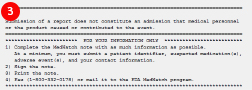
If you do not have an Adverse Drug Event template, you can download it on the RPMS EHR website. Talk to your Clinical Application Coordinator about adding the template to your EHR. - At the end of the note you will find the instructions for mailing or faxing the note to the FDA MedWatch program:
- Complete the note
- Print the note
- Sign the note
- Fax or mail the note to the FDA MedWatch program
Frequently Asked Questions about Submitting the FDA MedWatch Form
- Who is responsible for completing and submitting the ADE to the FDA?
- Any clinician can submit the FDA MedWatch form. Work with your staff to develop a local process for ensuring the submissions are completed and submitted.
- Are we allowed to send protected health information to the FDA?
- Yes. IHS clinicians are permitted under HIPAA to submit adverse drug events to the FDA MedWatch program. More information can be found on the FDA website .
- Do you need to include all of the information in the template to the FDA MedWatch program?
- No. You only need to submit the information that is relevant to the ADE. This includes the information about the ADE as well as information that may help the FDA better understand the potential risks or causes of the ADE. You can remove information that you deem not appropriate to include but you must always include at least these four items:
- A way to identify the patient
- Suspected cause of the ADE
- Description of the ADE that occurred
- Reporter information
- How soon does an ADE need to be submitted?
- There are not timelines on when ADEs must be submitted, but best practice is to collect, document, and submit the ADE as quickly as possible to promote capturing the data while it is fresh and to address potential ADEs early.


