Laboratory Requirements
Sites would need to ensure the following steps are completed prior to use of the Abbott ID NOW analyzer for patient testing of COVID-19:
- Ensure your site has a valid CLIA ceritificate on file.
- Notification to CMS and your clinic accrediting organization of intent to perform COVID-19 testing using the Abbott ID NOW analyzer under the FDA EUA for COVID-19.
- Creation of SOP for COVID-19 testing using the Abbott ID NOW analyzer. The SOP should encompass information pertaining to instructions for reporting and documenting results, notification of positive results, appropriate PPE usage and cleaning procedures, quality control, etc. All SOP's created need to be approved (signed) by the Laboratory Director before testing can begin.
- Reporting of results (per Abbott Instructions for Use) should include:
- Patient and Provider Fact Sheet
- Reporting positives to the state
- Reporting of false-positives and false-negatives and other significant deviations from performance characteristics to Abbott and FDA.
- Documentation of maintenance and temperature should be included in the SOP. Template log sheets can be found under the Abbott IFU, Test Product Insert, CLSI folder > 120006456 v01 ID NOW COVID-19 CLSI More Packet [Requires IHS Network Access] document.
- Reporting of results (per Abbott Instructions for Use) should include:
- Training of all users on the Abbott ID NOW analyzer using the 8 modules provided by Abbott and the IHS Training Video located in the "Training"[Requires IHS Network Access] section of the SharePoint.
- Competency of individuals need to be assessed using the appropriate requirements per your CLIA certificate, accrediting organizational requirements and/or local policy requirements. Abbott requires new users to perform 1 positive and 1 negative/blank test controls prior to testing. There are templates in the 'Documents" [Requires IHS Network Access] available to assist with competency.
- For waived sites: Personnel competency should adhere to accreditation requirements, including the frequency of performance noted.
- For non-waived sites: Personnel competency must be completed using the six (6) CLIA requirements for testing personnel competency.
- Competency frequency: Initial training, 6-months, and annually thereafter
- Appropriate PPE available for testing.
- PPE for COVID-19 testing includes:
- Gowns
- Gloves
- Face mask
- Eye protection or goggles
- Face shield or splash shield
- PPE for COVID-19 testing includes:
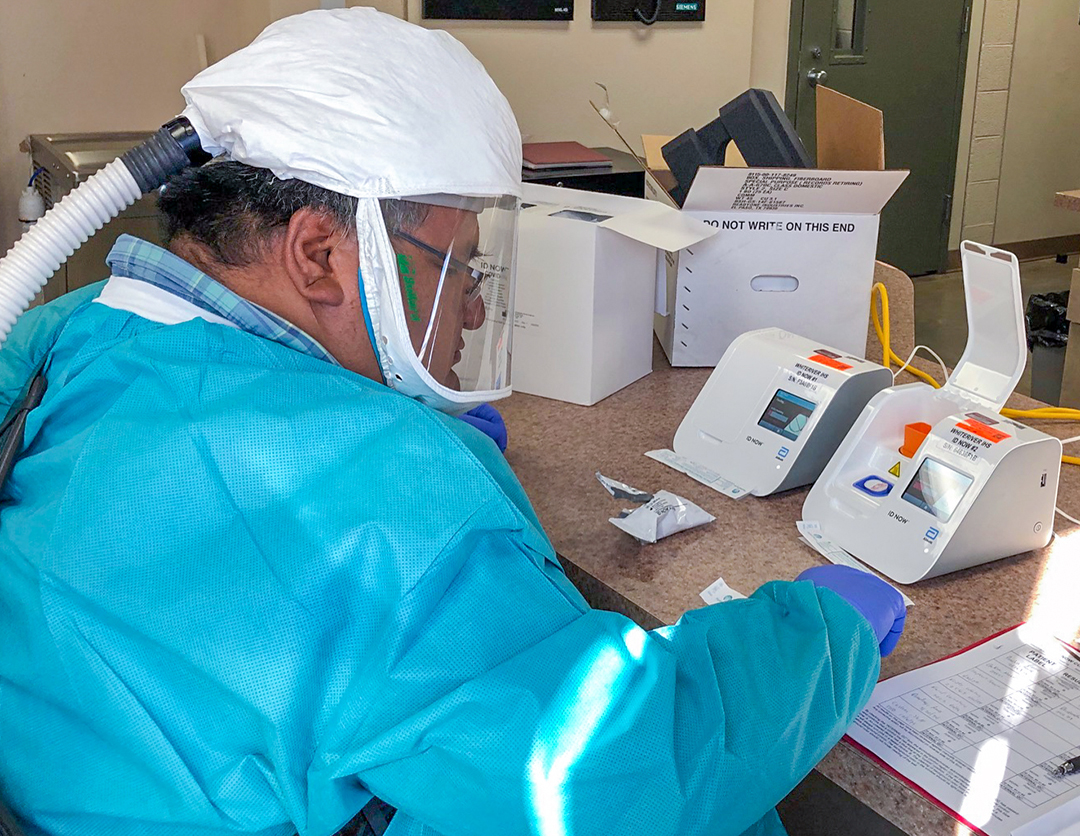
For diagnostic testing of specimens conducted outside of a BSL-2 laboratory, such as rapid respiratory testing performed at the point of care, use Standard Precautions to provide a barrier between the specimen and personnel during specimen manipulation.
For procedures with a high likelihood to generate aerosols or droplets, use either a certified Class II Biological Safety Cabinet (BSC) or additional precautions to provide a barrier between the specimen and personnel. Examples of these additional precautions include personal protective equipment (PPE), such as a surgical mask or face shield, or other physical barriers, like a splash shield.
- Testing location should be reviewed to ensure minimal traffic for testing location, separate room if possible, location should be near a sink and eyewash station.
- More CDC guidelines for COVID-19 can be found using the following links:
Note:
Standard Universal Precautions should be adhered to at all times
NLPC Resources
- ID NOW COVID-19 Patient Fact Sheet
- ID NOW COVID-19 HCP Fact Sheet
- Abbott IFU, Test Product Insert, CLSI
- Abbott SDS [Requires IHS Network Access]
- Abbott Technical Brief [Requires IHS Network Access]
- Shared templates - SOP, Training, and other implementation documents [Requires IHS Network Access]
SharePoint
Other Resources
- 120004872 v04 ID NOW Performance Best Practices [PDF - 305 KB]
- Abbott ID Now COVID 19 Training Form [PDF - 235 KB]
- Abbott ID Now Certificate of Training [PDF - 89 KB]
- Abbott ID NOW COVID19 Test Validation Form [PDF - 126 KB]
- COVID 19 Competency Assessment Mod High Complex [PDF - 347 KB]
- Laboratory Directors and Supervisors [PDF - 369 KB]
- Manual COVID 19 QC Patient Results Log [PDF - 216 KB]
- SCRSDS-0196 v4 Alere and ID NOW Positive and Negative Controls [PDF - 447 KB]
- SCRSDS-0275 v1 ID NOW COVID-19 Test Base SDS-US [PDF - 451 KB]
- TB000041 v1.0 Important Product Notice ID NOW COVID [PDF - 49 KB]
Note:
If you require assistance with the information on this
please contact Maureen James-Caires and Vanessa Vicenti.
- Abbott SARS CoV2 Test Procedure [PDF - 1.2 MB]
- Barcode Label COVID_19 QC Patients Results Log [PDF - 216 KB]
- ID NOW COVID-19 Technical Brief April 2020-Sample Type Labeling UpdateV2 [PDF - 151 KB]
- ID NOW COVID19 Test Procedure [PDF - 1.86 MB]
- IFU ID NOW COVID-19 [PDF - 3.22 MB]
- SARS CoV2 CDC Guidelines for Testing [PDF - 604 KB]
- SCRSDS-0274 v1 ID NOW COVID-19 Elution Buffer SDS-US [PDF - 567 KB]
- CLIA Good Laboratory Practice [PDF - 77 KB]
- NONWaived_Verification_Abbott_ID_NOW [PDF - 175 KB]
- Waived_Verification_Abbott_ID_NOW [PDF - 123 KB]


